Abstract
BACKGROUND
Noonan syndrome (NS) is one of several autosomal dominant multisystem disorders known as RASopathies. Common manifestations of NS include congenital heart defects and cardiomyopathy, lymphatic malformations, and predisposition to myeloproliferative disorders. Chylous fluid accumulation secondary to lymphatic malformations are seen in NS and are a major cause of morbidity and mortality often refractory to conventional medical management. There has been increasing interest in the use of pharmacologic MEK inhibition in the management of these patients given that activating RAS pathway mutations lead to downstream MEK activation that is causative of this pathology.
DESIGN/METHODS
Three patients with a confirmed diagnosis of NS are described. Each patient developed complications from chylous effusions refractory to conventional management and were subsequently enrolled on-study to treat with compassionate use oral trametinib from Novartis Pharmaceuticals on a single patient Investigational New Drug from the Food and Drug Administration (FDA). All patients were consented to be monitored for one year of therapy following a local protocol approved by the Colorado Institutional Review Board (COMIRB).
Patient 1: a 4-year-old female with NS due to a pathogenic germline mutation of the RIT1 gene [c.246T>G, p.Phe82Leu] born with severe hypertrophic cardiomyopathy, mitral valve dysplasia, and pulmonary valve stenosis. She developed bilateral chylous pleural effusions that were refractory to dietary modification, diuretics, octreotide, and sirolimus.
Patient 2: a 3-month-old female with NS due to a pathogenic germline mutation of the SOS1 gene [c.1322G>A; p.Cys441Tyr] born with esophageal atresia/tracheoesophageal fistula and moderate pulmonary valve stenosis. She developed bilateral chylous pleural effusions and ascites that were refractory to dietary modification and octreotide therapy.
Patient 3: a 4-month-old male with NS due to a gain-of-function mutation of PTPN11 [c.854T>C; p.Phe285Ser] with hypertrophic cardiomyopathy, pulmonary valve stenosis, respiratory insufficiency with suspected pulmonary lymphangiectasia, and persistent chylous pleural effusions in addition to Noonan syndrome-associated myeloproliferative disorder (NS-MPD) that had been refractory to traditional management.
RESULTS
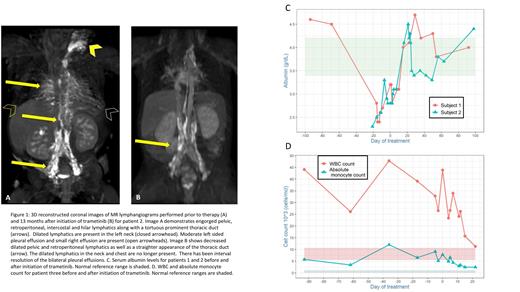
MEK inhibition with trametinib was used in three patients with NS and life-threatening complications with no medical or surgical treatment options. All three patients had dynamic contrast magnetic resonance lymphangiography (DCMRL) evidence of primary, central lymphatic dysplasia that manifested in lymphatic accumulation affecting cardiorespiratory function, nutrition, and the immune system. DCMRL imaging for patient 2 are highlighted in Figure 1 A and B. Within one month of initiating trametinib oral therapy, all three patients demonstrated response adequate to wean from mechanical ventilation and other supportive care modalities. Serum albumin levels improved as lymphatic leak resolved (Figure 1C). Patient 3 showed improvement in hypertrophic cardiomyopathy as evidenced by a decrease in both NT-proBNP and left ventricular mass by echocardiogram. Patients 1 and 2 demonstrated notable improvements in growth after one year of therapy, with increase in both weight and height percentiles. Patient 3 also presented with NS-MPD that responded with marked improvements in total WBC count as well as absolute monocyte count (Figure 1D).
DISCUSSION
Our experience adds to the growing body of evidence demonstrating the effectiveness of MEK inhibition on disease processes that are common in patients with NS and other RASopathies. None of the patients in our series experienced significant adverse effects from the medication aside from patient 2 who developed mild dermatitis. The efficacy of this therapy does not appear to be based on the underlying genotype, as each of the three patients we describe had different underlying molecular alterations (SOS1, RIT1, PTPN11). Substantial improvements in a variety of parameters including lymphatic malformations, cardiomyopathy, pulmonary valve stenosis, growth, and NS-MPD highlight the potential utility of trametinib in this patient population. Larger, prospective studies are necessary to confirm the efficacy of MEK inhibition and to assess the long-term safety of its use in this population.
Nakano: Novartis: Consultancy.
Trametinib is a MEK1/2 inhibitor that has been approved for the use in certain malignancies. Its off label use in children with Noonan Syndrome with significant lymphatic anomalies is based on the up regulation of the MAPK pathway in these patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal